Reducing Energy Costs During Salt Electrolysis with FLEMIONⓇ Fluorinated Ion Exchange Membranes

Reading Time: 2 minutes
Caustic soda (sodium hydroxide), caustic potash (potassium hydroxide), chlorine, and hydrogen-basic chemical are critical ingredients used in products all over the world. These chemicals are used in a wide variety of household and industrial products including soaps, cleaners, and electrolytes in batteries.
Electrolysis requires a significant amount of energy, so it’s critical to find any means to reduce energy consumption. Doing so reduces energy costs and operations costs while improving environmental responsibility. For more than 40 years, AGC has worked to reduce the electric resistance of fluorinated ion exchange membranes. Many chlor-alkali producers across the globe prefer the FLEMIONⓇ series of membranes because they have extremely low electrical resistance.
Substantial Energy Savings 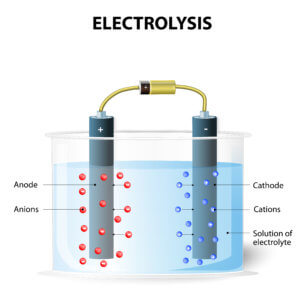
FLEMION fluorinated ion exchange membranes achieve substantial energy savings because they require less electrical current to decompose purified brine. According to Faraday’s law, the amount of any substance deposited or liberated during electrolysis is proportional to the quantity of electric charge passed and to the equivalent weight of the substance. A small part of the hydroxide ions (OH-) generated on the cathode (negative side) of the electrolyzer passes through the cation exchange membrane to the anode side and cannot be collected as products. By reducing this part as much as possible, the current efficiency increases. In addition, the use of carboxylic polymer helps these membranes achieve a continuous high electrical current efficiency of up to 98%.
FLEMION membranes require low voltage at high DC current to decompose brine in an electrolyzer. Oxidation at the anode occurs simultaneously with the reduction at the cathode, which makes current flow. Chlorine, caustic soda and hydrogen are all generated in the process.
High Impurity Resistance
Impurities like calcium (Ca) and magnesium (Mg) are byproducts of the brine used in the electrolysis process that can negatively impact the performance of a membrane. For example, Ca reduces current efficiency while Mg increases voltage. The membranes are also highly resistant to both chemicals and acids, including chlorine and caustic soda. They achieve both high ionic conductivity and ionic selectivity, are uniquely flexible and offer high mechanical strength and stability.
By investigating the influence of impurities on membranes, AGC was able to develop FLEMION membranes to achieve stable, long-term performance. Electrolysis plants in over 50 countries across the globe use them to achieve superior energy savings during salt electrolysis. AGC is committed to the continued advancement of the chlor-alkali industry. At our dedicated chlor-alkali lab located in Exton, PA, we provide testing services, analysis and results data that aids our customers’ continuous improvement efforts. For more information on how you can incorporate FLEMION membranes into your electrolysis operations, contact an AGC product expert.
 English
English